In today’s technology-driven world, the ubiquity of lithium-ion batteries is undeniable. From smartphones to electric vehicles and renewable energy storage systems, these batteries have become the cornerstone of portable power solutions. Yet, the magic behind their seamless performance lies in the intricate chemistry governing their operation.
The Essence of Battery Components
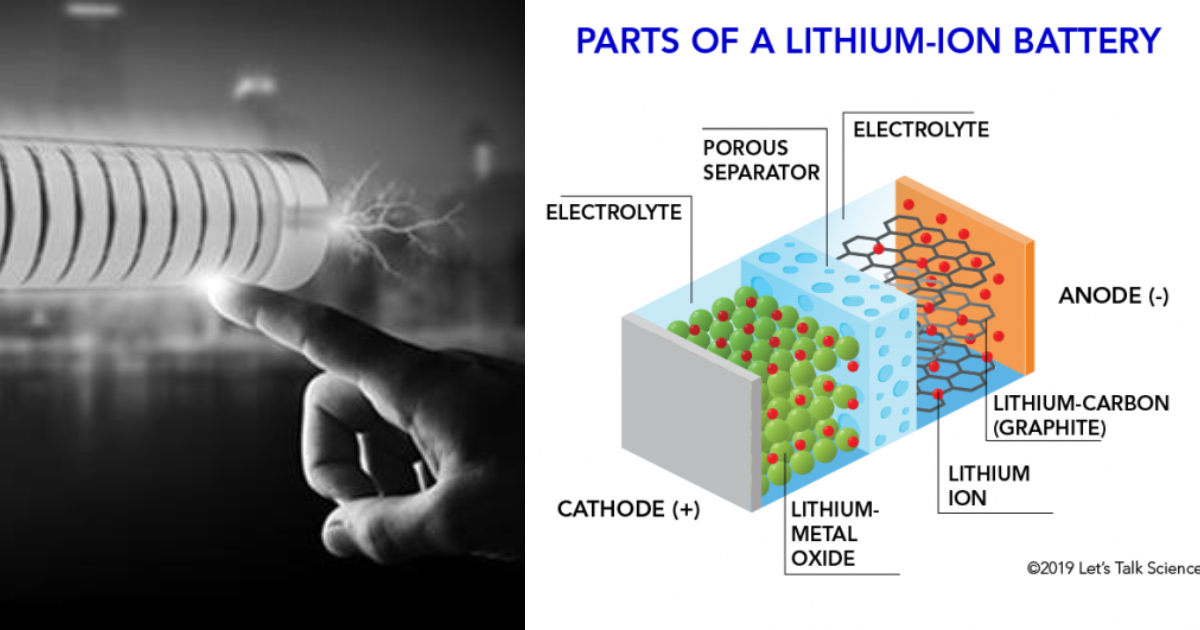
At the core of every lithium-ion battery are four fundamental components that work synchronously to store and release energy efficiently. The cathode, typically composed of materials like lithium cobalt oxide (LiCoO2), lithium iron phosphate (LiFePO4), or nickel manganese cobalt oxide (NMC), serves as the source of lithium ions during discharge cycles. Conversely, the anode, commonly made of graphite, silicon, or lithium titanate, absorbs these ions when the battery charges.
Facilitating the movement of ions between the cathode and anode is the electrolyte, a crucial conductor in the battery. It exists in various forms: liquid electrolytes, polymer electrolytes, and emerging solid-state electrolytes. Each type presents distinct advantages in terms of conductivity, safety, and stability. Moreover, the separator, a porous insulating material, plays a critical role in preventing electrical short circuits between the cathode and anode.
The Symphony of Charging and Discharging
The mesmerizing functionality of a lithium-ion battery unfolds during its charging and discharging processes. Charging involves the movement of lithium ions from the cathode to the anode via the electrolyte, facilitated by an external power source. Simultaneously, electrons flow through the external circuit, generating electrical energy that the battery stores for later use.
When the battery discharges, the reverse occurs: lithium ions migrate back to the cathode while releasing stored energy in the form of electricity. This cyclic movement of ions and electrons between the electrodes defines the core mechanism of a lithium-ion battery’s operation.
Cathode and Anode Chemistry: The Driving Forces
The performance and characteristics of a lithium-ion battery heavily rely on the choice of materials for the cathode and anode. The cathode’s material significantly influences factors such as energy density, stability, and cost. For instance, lithium cobalt oxide offers high energy density but comes with safety concerns, whereas lithium iron phosphate provides stability at the expense of slightly lower energy density.
Similarly, anode materials play a crucial role in determining battery capacity, charging rate, and lifespan. Graphite has long been a staple due to its stability and conductivity, but researchers are exploring silicon-based anodes to increase capacity, albeit with challenges related to expansion and stability during charge cycles.
Continued research and development in battery chemistry aims to identify and optimize materials that strike a balance between energy density, safety, cost-effectiveness, and environmental impact. Researchers are actively exploring advanced materials like solid-state electrolytes and novel cathode and anode compositions to address these challenges and unlock the full potential of lithium-ion batteries.
Power up your innovation with lithium-ion batteries from Akira, one of the leading Lithium-ion battery manufacturers. Experience reliability, performance, and sustainability in every charge. Contact us today to revolutionize your energy solutions and explore the future of power technology!